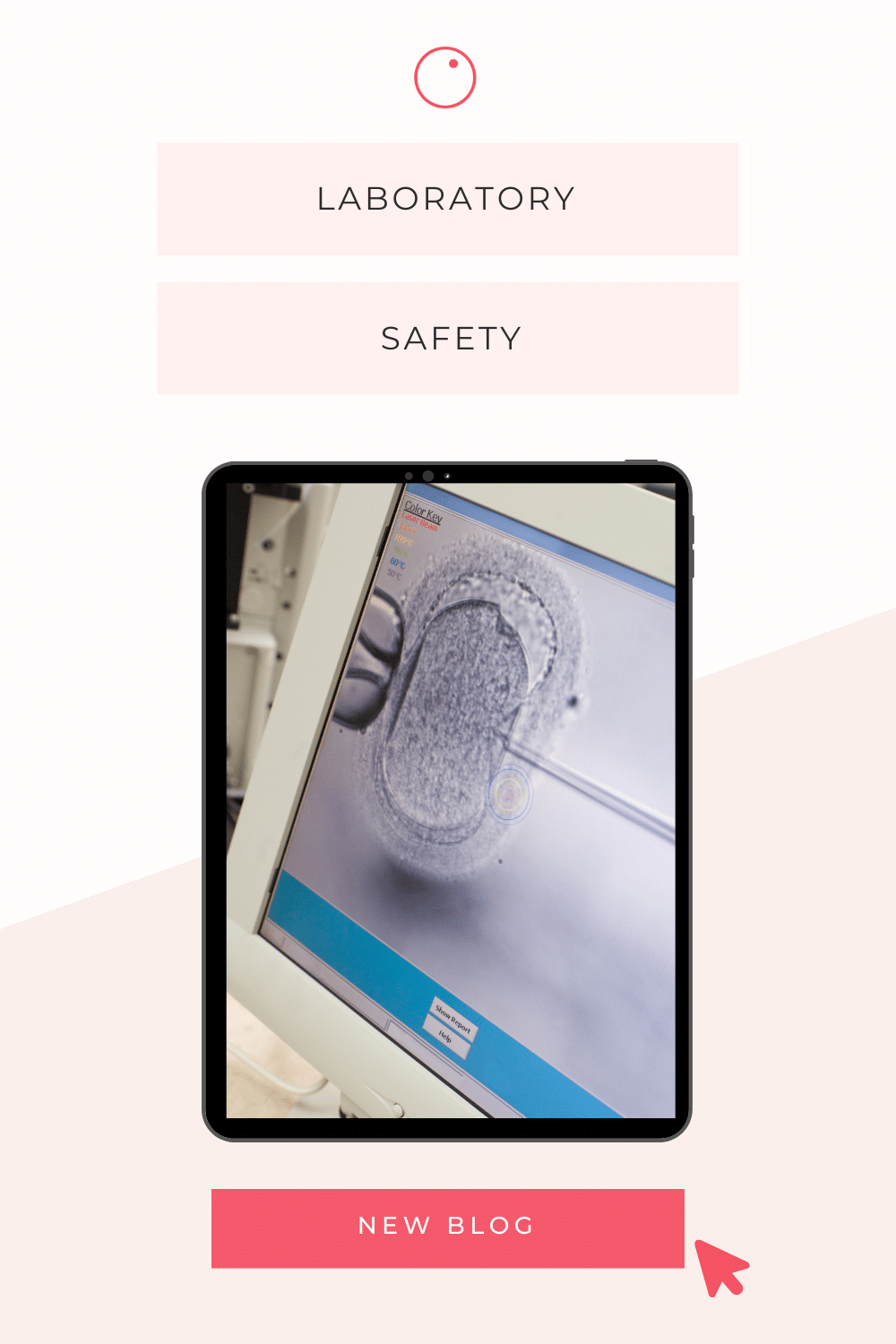
Embarking on the road to parenthood through fertility treatment is a journey of both anticipation and uncertainty. As you navigate this complex landscape, choosing the right destination for your fertility journey becomes crucial. In this blog article, we explore the intricacies of the Good Practice Guidelines for IVF Labs, providing you with invaluable insight into this critical aspect of your quest for parenthood.
Laboratory design: Creating an optimal environment
The foundation of any IVF laboratory is its design. Every aspect, from layout to infrastructure, is carefully considered to create an environment conducive to the delicate processes involved in assisted reproduction. Key considerations include:
Functional layout: The laboratory layout is designed to facilitate a seamless workflow, ensuring that each step of the IVF process - from gamete manipulation to embryo culture - is performed with precision and efficiency.
Environmental controls: Strict measures are taken to regulate temperature, humidity and air quality, creating an environment that is both stable and conducive to embryo growth and development.
Safety protocols: From bench design to ergonomic considerations, every element is tailored to minimise the risk of error and ensure the safety of laboratory personnel.

Ensuring pristine laboratory air quality
The quality of the air in the IVF laboratory is of paramount importance, as even minute contaminants can have a profound effect on the success of fertility treatments. Key considerations include:
Filtration systems: High-efficiency particulate air (HEPA) filters are used to remove airborne particles and microorganisms to protect the integrity of reproductive cells.
Controlled environments: Positive pressure systems and strict environmental controls minimise the risk of contamination and ensure that embryos are cultured in a sterile and controlled environment.
Continuous monitoring: Routine monitoring and maintenance protocols are implemented to ensure that air quality parameters remain within acceptable limits, with any deviations promptly addressed to maintain optimal conditions.


Laboratory equipment: Essential equipment and protocols
The success of IVF procedures depends on the availability of state-of-the-art equipment and adherence to strict protocols. Key considerations include:
Incubation systems: Incubators are meticulously calibrated to provide optimal conditions for embryo culture, with careful attention paid to factors such as temperature, humidity and gas composition.
Validation and calibration: All equipment undergoes rigorous validation and calibration procedures to ensure accuracy and reliability, with performance regularly checked to maintain consistency.
Safety measures: From gas supply systems to cryostorage units, each component is equipped with fail-safe mechanisms and alarm systems to mitigate the risk of equipment failure and ensure the safety of stored samples.
" Having personally visited all the clinics in Northern Cyprus, I have a thorough understanding of their laboratory facilities."
Cryopreservation equipment and materials: Ensuring reproductive potential
Cryopreservation has revolutionised the field of assisted reproduction, providing a means of preserving reproductive potential and expanding the options available to prospective parents. Key considerations include:
Safe storage practices: Cryostorage units are strategically located outside of the laboratory to minimise the risk of contamination, and stringent security measures are in place to ensure the integrity of stored samples.
Continuous monitoring: Routine monitoring and alarm systems are implemented to detect any deviations in temperature or liquid nitrogen levels, with immediate action taken to address any anomalies and prevent loss of stored material.
Staff training: Staff are trained in the safe handling and storage of cryogenic materials, with strict protocols in place to minimise the risk of exposure and ensure the safety of laboratory staff.
Reducing the risk of infectious agents
The handling of biological materials in the IVF laboratory carries inherent risks of cross-contamination and transmission of infectious agents. Key considerations include:
Screening protocols: Strict screening protocols are implemented for both patients and laboratory staff to minimise the risk of transmission of infectious diseases, with vaccination against diseases such as Hepatitis B recommended for all staff.
Safety measures: Strict adherence to hygiene protocols and aseptic techniques minimises the risk of cross-contamination, and protective measures such as gloves, masks and laboratory clothing further protect against exposure to infectious agents.



Implement protective measures for personnel and materials
Maintaining a safe and sterile environment in the IVF laboratory requires a comprehensive approach to staff training and facility management. Key considerations include:
Hygiene practices: Strict adherence to hygiene protocols, including hand washing and the use of protective clothing, minimises the risk of contamination and ensures the integrity of biological materials.
Waste management: Proper disposal of potentially infectious materials is essential to prevent the spread of disease, with strict protocols for waste segregation and disposal.
Continuing education: Ongoing training and education is provided to laboratory staff to ensure compliance with safety protocols and to promote a culture of safety within the laboratory.
Conclusion
In the quest for parenthood, the IVF laboratory is a beacon of hope, where dreams are nurtured and new beginnings take root. By adhering to good practice guidelines, fertility clinics uphold the highest standards of safety and efficacy, guiding you on your journey to parenthood with confidence and peace of mind.
Your journey to parenthood is our top priority, and we are committed to providing you with the guidance and support you need. Together, we will navigate the complexities of fertility treatment with compassion, expertise and unwavering dedication.
Source
EUROPEAN SOCIETY OF HUMAN REPRODUCTION AND EMBRYOLOGY